Struggling to crystallize (not really about crystals)
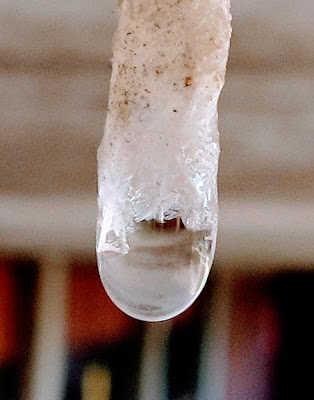
One of the attractions of my daily ride to work is where I hop off my bike and ascend the steps of the library plaza. As I carry my bike up the stairs, I get a pretty good view of the underside of the plaza. Like all flat roofs the plaza leaks, and where the water emerges below, dissolved minerals form soda-straw stalactites just like inside a cave. They never get very big; students see them and break them off, but it is fascinating to watch them form.
In the detail shot at right you can actually see emergent crystals – calcite, probably. The coloration of this stalactite changes halfway along its length, representing the end one month ago of heavy snow; (and the salt that is scattered around after clearing it) less salt means less rust from the reinforcing rods of the concrete plaza, so the deposited minerals shift from reddish-brown to pearly white.
Mineral crystals seem like ordinary things and they are, until you think about what’s going on as they form. Calcite forms 74° 55′ crystal surfaces, resulting from the molecular properties of calcium carbonate, which in turn emerge from deeper atomic properties. As the phase change from dissolved molecules to crystal occurs, the molecules align just so. Just because something is ordinary doesn’t mean that, if you look at it closely enough, it isn’t wonderful.
Every dissolved mineral crystallizes differently, if at all. Pressure and temperature affect the result: one of the crystalline forms of carbon forms a transparent octahedral lattice that is popular with young couples in love. Or consider calcium oxalate; in complex urinary chemistry it forms jagged, pointy crystals that cause larger accumulations of it to lodge in your ureter. Now you’re on the floor writhing in pain, hoping for some morphine or if not, then hoping someone will just shoot you in the head.
Conditions have to be just right for crystals to form. If the concentration is wrong, no crystals. If several elements and compounds are dissolved, again crystallization may not happen, or it may result in various muddy interactions between tiny crystalline formations. But if you want one clear, recognizable crystal, the concentration and purity have to be within a certain range.
That’s been my problem the last few weeks; too many dissolved elements and I can’t seem to get the right concentration for anything to crystallize. Some of the elements: Russia has contracted with British Petroleum to explore for Arctic oil. We’re attacking Lybia. Republicans seem intent on rolling us back to the Robber Baron era. We have awesome pictures of the planet Mercury now. And thanks to a substandard Japanese power company in denial about tsunami waves, “clean coal” fantasists have a new pet argument. Republicans again: women in several states will have to answer to bureaucrats if they want an abortion. Big dreams are passé; as our country rusts away, no politician on either side of the aisle seems to have the courage to suggest openly that we invest in infrastructure or education. The AFA’s Bryan Fischer lumps gays, atheists, Muslims and liberals generally into one big evil gooey “Them”, which wouldn’t matter if US Senators and Congressmen didn’t curry his favor. And practically everybody is in denial about Peak Oil.
Add to all that I just have not been feeling very well lately, so concentration has been a problem. It’s tempting to disengage; to concentrate only on my own, parochial concerns and simply ignore the larger world. But I am not wired that way. For one thing, issues we perceive as “local” are, like the crystals forming as water evaporates at the end of a calcite tube, only the business end of something much larger and more complex. So I will keep trying.

This is a fine writing about crystals, and lovely photos.
All in all, a good blog post.
But what impressed me most happened later, when we were walking on the path, and you pointed out the little item of interest.
It’s a wonder you noticed at all!
the dang thing is only 4″ dripped down from the concrete!